Symbol:3pinwkzgpvg= Carbon
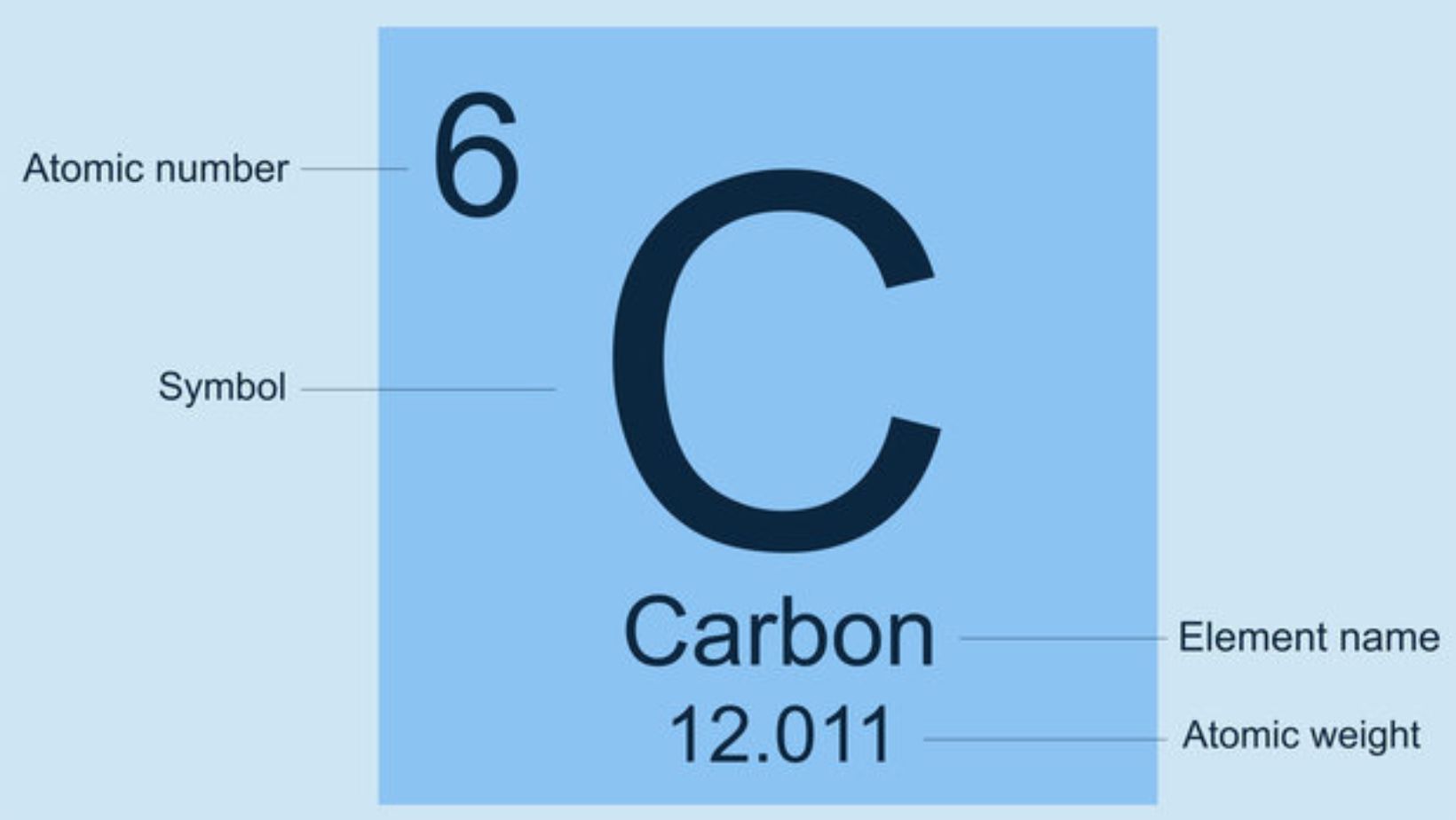
Carbon, symbolized by the letter ‘C’ on the periodic table, is a cornerstone of life and industry. As a fundamental building block of organicchemistry, carbon forms countless compounds, making it essential in fields ranging from biology to materials science. Its unique ability to bond with itself and other elements creates the backbone for complex molecules like proteins and DNA, which are crucial for life.
Beyond its biological significance, carbon plays a pivotal role in technology and energy. From the graphite in pencils to the diamonds in jewelry, carbon’s versatility is unmatched. It’s also at the heart of discussions on climate change, with carbon emissions being a major focus for environmental policies worldwide. Understanding carbon’s properties and applications not only enriches our knowledge of the natural world but also equips us to address pressing global challenges.
Understanding Symbol Carbon

Symbol carbon, identified by the letter ‘C’ and atomic number 6, is a fundamental element in the periodic table. As a non-metal, carbon possesses unique properties, allowing it to form multiple allotropes like graphite, diamond, and graphene. Each allotrope displays distinct physical characteristics due to variations in carbon atom arrangements. Graphite is a good conductor of electricity and has lubricatingproperties, while diamond ranks as the hardest natural material known. In contrast, graphene exhibits remarkable strength and conductivity, making it a promising material for technological advancements.
Carbon’s ability to form four covalent bonds enables the formation of diverse organic compounds. These compounds include hydrocarbons, alcohols, and carboxylic acids, which are crucial in various industries. Hydrocarbons serve as primary energy sources in fuels like gasoline and natural gas. Alcohols are solvents in pharmaceuticals and chemical reactions, while carboxylic acids contribute to the production of plastics and food preservatives.
In environmental science, the carbon cycle illustrates carbon’s dynamic role in ecosystems. Carbon dioxide, a greenhouse gas, impacts global temperatures and climate patterns, emphasizing the need for sustainable practices. Efforts to minimize carbon emissions focus on renewable energy sources, carbon capture technologies, and international policies.
Understanding symbol carbon is essential in fields ranging from chemistry to environmental science, reflecting its comprehensive influence on life and technology.
Historical Background of Carbon Symbol
Carbon’s symbol, ‘C’, traces its origins to ancient languages. The name derives from the Latin “carbo,” meaning coal. Within civilizations using Latin, coal was integral to heating and metalworking processes. This foundational importance led to the designation of carbon with ‘C’ in the modern periodic table.
In the late 18th century, French chemist Antoine Lavoisier identified carbon as an element. Through systematic experiments, he demonstrated its combustion properties and categorized it alongside other fundamental substances known at the time. The symbol ‘C’ emerged during efforts to standardize chemical nomenclature, aligning with symbols for other elements like hydrogen (H) and oxygen (O).
As chemistry evolved in the 19th century, the symbol ‘C’ gained consistent usage in scientific communities. It became prominent in publications, aiding both educational endeavors and industrial advancements. Carbon’s pivotal role in organic chemistry further cemented its symbolic representation, especially as chemists began forming molecular structures, emphasizing carbon’s tetravalency.
By aligning with scientific standards and historical context, the carbon symbol ‘C’ underscores the element’s universal importance. It links modern chemical understanding to its historical roots, reflecting carbon’s enduring legacy in both ancient and contemporary contexts.
The Chemistry of Carbon
Carbon has an atomic number of 6. Its atomic structure consists of a nucleus containing six protons and six neutrons surrounded by sixelectrons. Electrons occupy two energy levels: two electrons in the inner shell and four in the outer shell. This configuration allows carbon to form up to four covalent bonds with other atoms, making it highly versatile in creating complex molecules. The ability to form single, double, and triple bonds results in diverse carbon-based compounds.
Carbon exists in several isotopes, with carbon-12 and carbon-13 being stable and most common. Carbon-12 accounts for approximately 98.9% of natural carbon and plays a standard role in chemical calculations and atomic mass. Carbon-13 makes up about 1.1% and serves as a key isotope in NMR spectroscopy for structural analysis. Carbon-14, a radioactive isotope, has a half-life of about 5,730 years and is crucial in radiocarbon dating for determining the age of archaeological finds and geological formations.



